Notes
Nucleic Acids: Sugars & Bases
Sections
Overview
This tutorial provides an engaging way to master material requisite for a standard Biology/Biochemistry course (primary references: Lehninger Principles of Biochemistry and Molecular Biology of the Cell).
We provide board preparation (USMLE/COMLEX, MCAT, AP Biology, etc...) questions at the end for focused review of key topics.
dna
DNA components
- Phosphate
- Nitrogenous base: 1 of 4 potential bases (adenine, guanine, cytosine, thymine)
- Deoxyribose
rna
RNA components
- Phosphate
- Nitrogenous base: 1 of 4 potential bases (adenine, guanine, cytosine, uracil)
- Ribose
Differences between DNA and RNA
DNA has deoxyribose. RNA has ribose. DNA uses thymine. RNA uses uracil. Ribose has an hydroxyl at carbon 2, deoxyribose does NOT.
Purines & Pyrimidines
Purines have 2 rings. Think: "Pure As Gold" for Purine = Adenine and Guanine
- Guanine (G)
- Adenine (A)
- Double-ring structure with 4 nitrogens and 4 double bonds.
Pyrimidines have 1 ring. Think: "Single CUT" for Pyrimidines have a single ring. CUT stands for cytosine, uracil (RNA), thymine (DNA). Thymine is Methy*lated (methylation of uracil creates thymine).
- Cytosine (C)
- Thymine (T) DNA only
- Uracil (U) RNA only
- One ring structure with 2 nitrogens and 3 double bonds.
Chagraff's rule
According to Chargaff's rule, in DNA, the amount of one base (the purine) equals the amount of its paired base (the pyrimidine).
- The amount of guanine = the amount of cytosine
- The amount of adenine = the amount of thymine
As an example, if DNA is 30% guanine, then it is also 30% cytosine (total: 60%). Thus, out of 40% remaining (100% - 60%), 20% will be adenine and 20% will be thymine.
Hydrogen Bonds
3 hydrogen bonds between G and C
- The amino group hydrogen on C2 of guanine bonds with the carbonyl oxygen on C2 of cytosine
- The hydrogen atom bound to N1 of guanine bonds with N3 of cytosine.
- An amino group hydrogen on C4 of cytosine bonds with the carbonyl oxygen on C6 of guanine.
2 hydrogen bonds between A and T/U
- The hydrogen atom on N3 of thymine/uracil bonds with N1 of adenine
- An amino group hydrogen on C6 of adenine bonds with the carbonyl oxygen on C4 of thymine/uracil
Thus the bonding between G and C is stronger than that of A and T (3 bonds vs 2 bonds).
Introduction to Nucleic Acids
Overview
Here we will learn about about the structures of the sugars and nitrogenous bases that constitute nucleic acids and about base pairing.
Nucleic acids
There are two types of nucleic acids:
- DNA (deoxyribose nucleic acid)
- RNA (ribose nucleic acid)
- They are distinguished by the bases and the types of sugars that comprise them.
- Nucleotides are the building blocks of nucleic acids (DNA and RNA).
- Each nucleotide has three components
- Nitrogenous base
- Sugar
- At least one phosphate group.
DNA
- DNA's components are:
- deoxyribose (which is its sugar)
- 1 phosphate
- 4 bases
RNA
- RNA's components are:
- ribose (which is its sugar)
- 1 phosphate
- 4 bases
The 4 Bases of DNA & RNA
- For both DNA and RNA, 3 of the 4 bases are:
- Adenine
- Cytosine
- Guanine
- For DNA, the 4th base is:
- Thymine.
- For RNA, the 4th base is:
- Uracil.
Nucleotide Sugars
Now let's draw the sugars and bases of a nucleotide.
DNA & RNA
We begin with the sugars:
- Deoxyribose (of DNA)
- Ribose (of RNA)
- As we'll see, deoxyribose has one less hydroxyl group than ribose, hence its name deoxy-ribose.
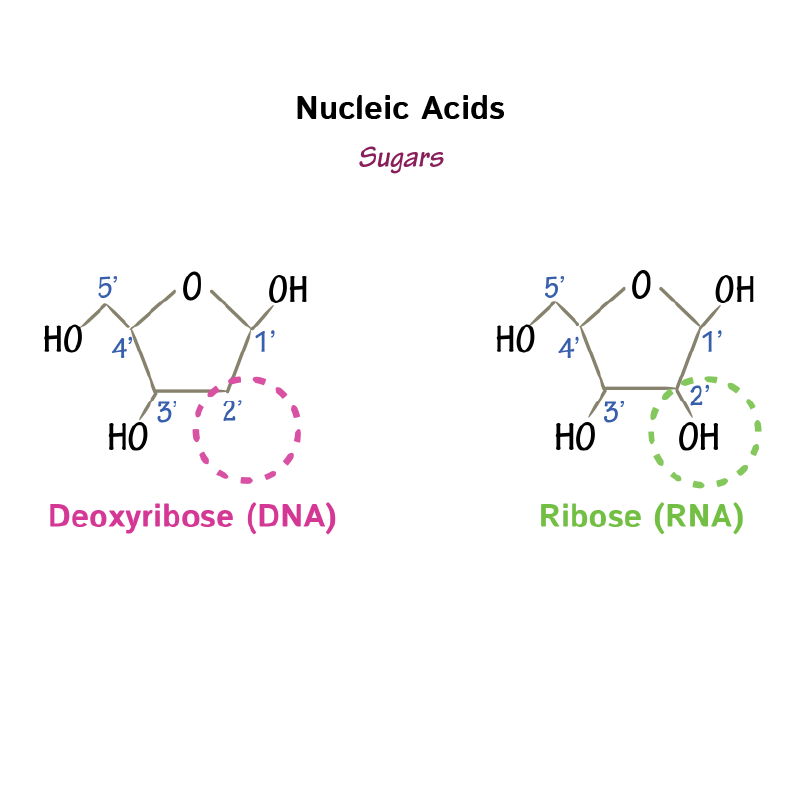
Deoxy-ribose
- To draw deoxyribose, draw a pentagon with an oxygen atom inserted at the top.
- Label carbons 1' through 4' going clockwise from the oxygen atom.
- Now add carbon 5' as an attachment to carbon 4'.
- Next, let's add hydroxyl groups to carbons 1', 3' and 5'.
Ribose
- To draw ribose, draw a pentagon with an oxygen atom inserted at the top.
- Label carbons 1' through 4' going clockwise from the oxygen atom,
- Add carbon 5' as an attachment to carbon 4'.
- Finally, add hydroxyl groups to carbons 1', 2', 3', 5'.
- Specify that whereas ribose has an hydroxyl at carbon 2', deoxyribose does NOT.
Nitrogenous bases
Now let's draw the nitrogenous bases.
- There are two general categories of nitrogenous bases in nucleic acids:
- Purines
- Pyrimidines
- For each category, we will first draw their general structures and then the bases that are part of this group.

Purines
- Purines are guanine (G) and adenine (A).
Pyrimidines
- Pyrimidines are cytosine (C), thymine (T) and uracil (U).
purine structure
- For the general structure of a purine, draw a hexagon.
- Label positions 1 through 6 going counterclockwise starting to the left of the top of the hexagon.
- Insert a nitrogen atom at position 1.
- Then, position 3.
- Now add a double bond between N1 and C6.
- Add another double bond between C2 and N3.
- Add a third double bond between C4 and C5
- Next, at positions 4 and 5 add a second, five-membered ring: it's shaped like a pentagon.
- Now going clockwise from the top of the pentagon, label positions 7, 8 and 9.
- Insert a nitrogen atom at position 7.
- Then, position 9.
- Now add a double bond between N7 and C8.
- Finally, add a hydrogen atom to N9.
The Purines
As we begin to draw the purines, keep in mind that they all have four double bonds: we can use this to check our structures.
Adenine
- Redraw our general purine structure with its 4 double bonds but add an NH2 group to C6.
Guanine
Next, let's draw guanine.
- Redraw the general purine structure, but without the double bonds.
- Add a hydrogen atom to N1.
- Add an NH2 group to C2.
- Add a double-bonded oxygen to C6.
- Now let's add the 3 other double bonds:
- Between C2 and N3
- Between C4 and C5
- Between N7 and C8
The Pyrimidines
Now, let's turn our attention to the pyrimidines.
For the general structure of a pyrimidine, draw a hexagon.
- You'll notice artificial breaks in the hexagon that we will fill in momentarily.
- Label positions 1 through 6, going counterclockwise, beginning at the bottom of the hexagon.
- Insert a nitrogen atom at position 1
- Then, at position 3
- Now add a double bond between N1 and C2
- Add another double bond between N3 and C4
- Add a third double bond between C5 and C6
- Indicate that the pyrimidine structure is similar to the hexagonal ring of purine.
- As we begin to draw the pyrimidines, keep in mind that they all have three double bonds.: we can use this to check our structures.
Cytosine
- Redraw our general pyrimidine structure without the double bonds.
- Add a hydrogen atom to N1
- Add a double-bonded oxygen to C2
- Add an NH2 group to C4
- Now let's add the 2 other double bonds as follows:
- Between N3 and C4
- Between C5 and C6
Thymine
- Redraw our general pyrimidine structure, but without the double bonds.
- Add a hydrogen atom to N1
- Then, N3.
- Now, add the 3 double bonds:
- Add a double-bonded oxygen to C2
- Then, to C4
- Now add a double bond between C5 and C6
- Next, add a methyl group to C5: We'll see how it makes thymine unique to DNA
- Write that thymine is found in DNA (Not RNA).
Uracil
- Redraw thymine, but omit the methyl group at C5.
- Indicate that it is unique to RNA; it's not found in DNA.
- Specify that the sole difference is that uracil lacks the methyl group, which is found on thymine.
Scientific/Clinical Application
Under normal circumstances, uracil is not found in DNA. It is structurally similar to thymine, but lacks a methyl group at carbon 5. This difference is critical.
- Cytosine can spontaneously deaminate (lose an amino group), converting into uracil. If this happens in DNA, the presence of uracil indicates a mutation, and must be corrected.
- Thus, DNA uses thymine instead of uracil to help DNA repair enzymes distinguish between normal bases and damaged cytosine.
- Enzymes like uracil-DNA glycosylase scan the DNA and remove uracil when it is found, restoring the correct cytosine–guanine base pair.
- If uracil were normally present in DNA, this distinction would be lost, and the cell would be unable to recognize and fix certain mutations—increasing the risk of genetic errors and cancer.
DNA Structure
Finally, we will show how the bases can form base pairs with each other to create the double-stranded structure of DNA.
Adenine and thymine pairing
- Write that adenine and thymine pair with each other via hydrogen bonds.
- Show that they pair as follows:
- The hydrogen atom on N3 of thymine bonds with N1 of adenine.
- An amino group hydrogen on C6 of adenine bonds with the carbonyl oxygen on C4 of thymine.
Cytosine and guanine pairing
- Write that cytosine and guanine pair with each other via hydrogen bonds.
- Show that they pair as follows:
- An amino group hydrogen on C2 of guanine bonds with the carbonyl oxygen on C2 of cytosine.
- The hydrogen atom bound to N1 of guanine bonds with N3 of cytosine.
- An amino group hydrogen on C4 of cytosine bonds with the carbonyl oxygen on C6 of guanine.
Numbering (Priming)
Please be advised that the final image has been updated to reflect that by standard convention, the carbon atoms of the pentose sugar in nucleotides are designated with a prime ('), thus their numbering is 1', 2', 3', and so forth, whereas the numbering of the carbon atoms in the base does not contain the prime designation.
Consider that the directionality of DNA strands as being: 5' to 3' or 3' to 5' refers to the carbon atom sugars.
- The prime designation is not yet a part of this video tutorial.
BOARD QUESTIONS (USMLE, MCAT, AP, etc...)
These questions will help focus your board review. Considerable overlap exists in what is highlighted on the various exams. USMLE/COMLEX students should use the other exam questions as a helpful reinforcement of key material and to foster rapid recall.
For international students, the information in this tutorial is relevant to any exams that require basic science knowledge including (but not limited to): Staatsexamen (1), ECNi, MIR, Esame di Stato, AMC CAT MCG, FMGE, MCCQE (1).
Overlapping high yield topics:
- Differences between DNA & RNA (uracil (RNA) vs thymine (DNA))
- Application of Chargraff's rule
- Effect of triple hydrogen bonding
- Impact of the 2' hyrdoxyl