USMLE/COMLEX 3 - Adrenal Insufficiency
Start your One-Week Free Trial
Already subscribed? Log in »
Here are key facts for USMLE Step 3 & COMLEX-USA Level 3 from the Adrenal Insufficiency tutorial, as well as points of interest at the end of this document that are not directly addressed in this tutorial but should help you prepare for the boards. See the tutorial notes for further details and relevant links.
- --
VITAL FOR USMLE/COMLEX 3
Emergency Recognition and Management
1. Adrenal insufficiency is associated with high morbidity and mortality, which increase when diagnosis is delayed.
2. Be suspicious of adrenal crisis in patients who present with acute shock that is refractory to vasopressors and fluid replacement.
3. Acute adrenal crisis occurs when patients with adrenal insufficiency face additional stressors, including infections, trauma, surgery, and dehydration.
4. Acute adrenal crisis presents with shock, fever, dehydration, nausea, vomiting, hypoglycemia, apathy, and weakness.
5. In bilateral adrenal hemorrhage, signs include fever, nausea, vomiting, tachycardia, cyanosis, and flank and abdominal pain and tenderness.
Diagnostic Approach
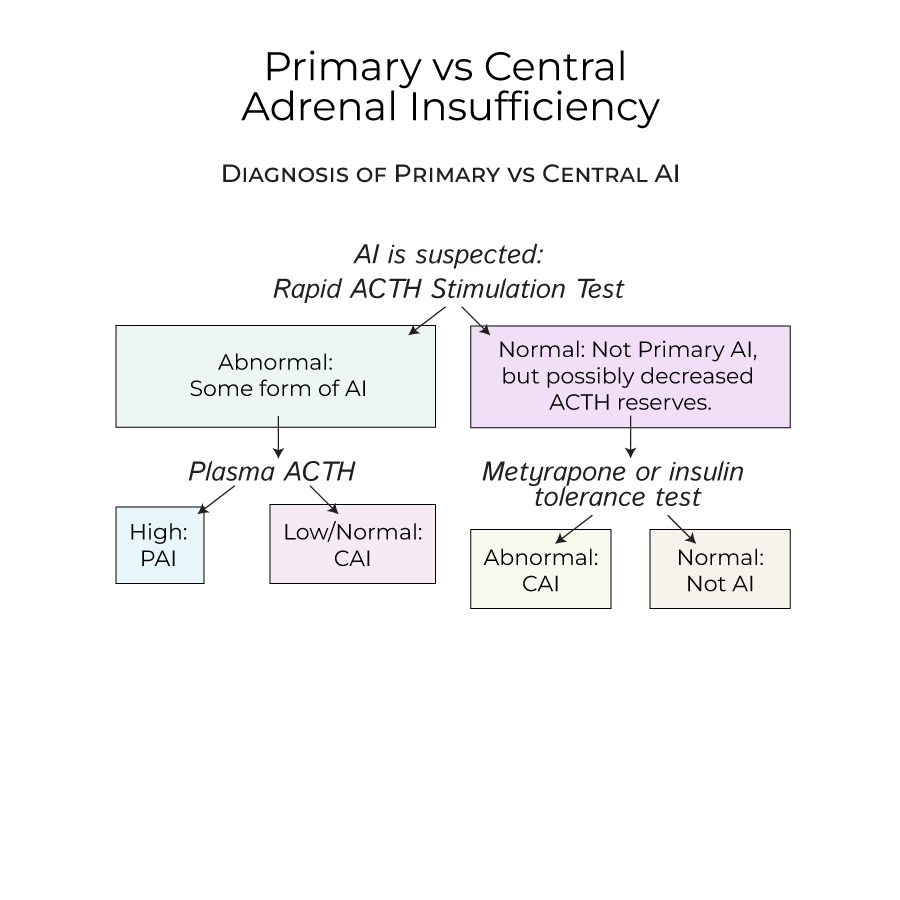
1. When AI is suspected, a rapid ACTH stimulation test is performed, which would raise cortisol levels in a healthy person.
2. If cortisol levels are abnormal after ACTH stimulation, measuring plasma ACTH determines the type of AI: high ACTH indicates Primary AI; low/normal ACTH indicates Central AI.
3. Normal ACTH stimulation test results may still reflect decreased ACTH reserves in early stages of central AI, requiring additional testing.
4. To test for early central AI, administer metyrapone (which prevents cortisol synthesis) or an insulin tolerance test, and measure cortisol levels.

Treatment Strategies
1. Treatment for AI includes glucocorticoid replacement, and, in the case of primary AI, mineralocorticoid replacement.
2. To avoid acute adrenal crisis in diagnosed patients, provide additional doses of glucocorticoids when stressors, such as surgery, are expected.
3. Beware of overtreatment, especially with glucocorticoids, as excessive cortisol exposure produces Cushing's syndrome.
4. Patients should carry medical cards that warn of their condition in case of accidental trauma.
- --
HIGH YIELD
Special Patient Populations
1. Primary AI affects slightly more women than men and is typically diagnosed in patients 30-50 years old.
2. In children, watch for Waterhouse-Friderichsen Syndrome, with adrenal bleeding caused by septicemia, most often associated with meningococcal or pseudomonas infections.
3. In children, recurrent mucocutaneous candidiasis is a potential indicator of APS-1, manifesting as thrush and angular cheilitis.
4. Patients with APS are likely to have additional endocrine-related disorders.
Risk Assessment and Prevention
1. Risk factors for bilateral adrenal hemorrhage include anti-coagulation therapy and disorders that increase the risk of venous blood clots, such as antiphospholipid antibody syndrome and systemic lupus erythematosus.
2. Central AI may develop from sudden cessation of steroids or inability of the HPA axis to respond to additional stressors.
3. The anatomy of the adrenal gland blood supply (three arteries but one vein) makes it especially vulnerable to venous congestion and hemorrhaging.
Clinical Differential Diagnosis
1. In chronic AI, adrenal reserves may initially maintain basal hormone levels, but with impaired ACTH stress response, complicating diagnosis.
2. Primary AI presents with hyperpigmentation, hypotension, hyponatremia, hyperkalemia, and metabolic acidosis.
3. Central AI manifestations are similar to Primary AI, with important exceptions: no hyperpigmentation (since ACTH is deficient) and less hypovolemia/hypotension (since aldosterone secretion is normal).
4. Chronic Primary AI symptoms include weakness, fatigue, weight loss, gastrointestinal problems, and possibly salt cravings.
5. Central AI symptoms are chronic and nonspecific due to glucocorticoid deficiencies.
Etiology-Specific Management
1. Most common cause of Central AI is long-term exogenous steroid use.
2. In the United States, autoimmune diseases are the most common causes of primary AI, with anti-adrenal antibodies destroying the adrenal cortex.
3. Drugs that interrupt ACTH production include immune checkpoint inhibitors, high-dose progestins, and opioids.
4. Additional causes of Primary AI include adrenoleukodystrophy, infiltrative disorders, familial glucocorticoid deficiency, congenital adrenal hypoplasia, cortisol resistance, drug effects, and disorders of aldosterone synthesis & action.
- --
Beyond the Tutorial
Clinical Decision Making
1. Consider a stress-dose steroid protocol for all patients with known adrenal insufficiency undergoing procedures or experiencing acute illness.
2. When treating adrenal crisis, administer hydrocortisone before obtaining confirmatory tests if clinical suspicion is high.
3. Avoid abrupt discontinuation of glucocorticoid therapy in patients who have been on long-term treatment.
4. Address fluid and electrolyte imbalances concurrently with hormone replacement in acute presentations.
Long-term Management
1. Establish individualized replacement regimens based on clinical response rather than laboratory values alone.
2. Implement a tapering schedule when discontinuing long-term glucocorticoid therapy to allow for HPA axis recovery.
3. Educate patients on self-adjusting glucocorticoid doses during illness and stress.
4. Monitor for complications of chronic glucocorticoid therapy, including osteoporosis, glucose intolerance, and cardiovascular risk.
Systems-Based Practice
1. Establish clear communication with emergency services about management of patients with adrenal insufficiency.
2. Provide patients with emergency kits containing injectable hydrocortisone and detailed instructions.
3. Coordinate care with endocrinologists for optimal long-term management.
4. Consider screening family members for autoimmune adrenal disorders when genetic predisposition is suspected.
